Leading Cell Therapy Manufacturing
Project Overview
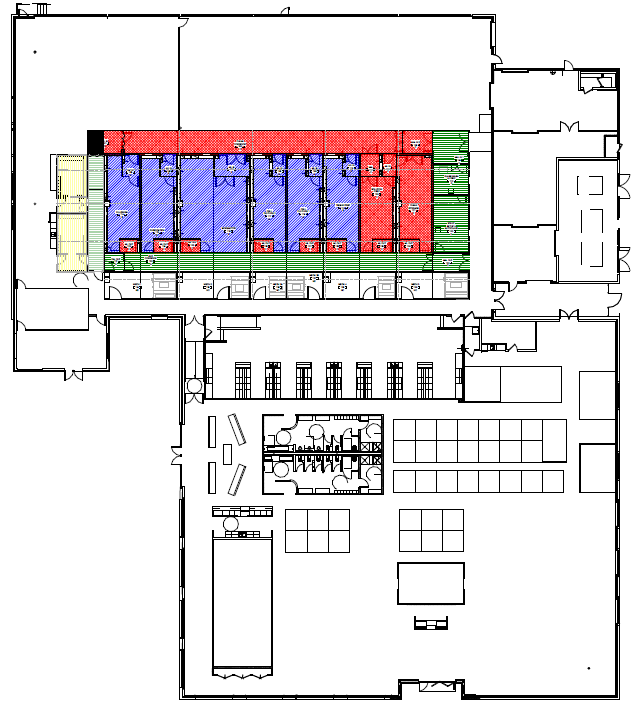
This new mesenchymal stem cell therapy facility was constructed as part of a renovation of a historic ice storage building. The facility was designed and constructed for Phase III and commercial launch production. The program included 6,500 square feet of cleanroom production, equipment sterilization and staging, tissue sampling and handling, cell culture, purification, fill-finish, and solution preparation suites. GBA led the process discovery, developed conceptual layouts, assisted with equipment engineering services, and provided the design for the production suites and the host facility’s utility upgrades. GBA’s process engineers used these findings to assist in the process train development and layout of the cleanrooms.
The architectural and MEP team provided the design for the cleanroom pods where production would take place. Mechanical and electrical design was completed to support production and utility areas of the facility.